
 Document
Document
Better AWR Outcomes. Reinforced by Data. Critical healing period and repair strength
GORE® BIO-A® Tissue Reinforcement provides a unique 3D tissue-building scaffold that elicits a specific tissue response during the critical wound healing period. Video
Video
Trends, Tools, Trials: Complex Abdominal Wall Repair in 2020
Matthew I. Goldblatt, M.D., FACS, discusses a case study of a patient undergoing treatment for simultaneous colon and rectal cancers and had a prior low anterior colon resection procedure with a diverting ileostomy. Video
Video
Laparoscopic Ventral Hernia Repair using GORE® SYNECOR® BioMaterial
Carl R. Doerhoff, MD, FACS, practices in SurgiCare in Jefferson City, Missouri, presents the use of the GORE® SYNECOR® BioMaterial Laparoscopic ventral hernia repair. Document
Document
Better AWR Outcomes. Reinforced by Data. Confidence for surgeons and patients in ventral hernia repair
GORE® BIO-A® Tissue Reinforcement is designed as an alternative to longer-term resorbable and permanent meshes to offer a strong repair while avoiding risk for long-term mesh-related complications Document
Document
Bioabsorbable Mesh for Hernia Repair: Know your Options
GORE® BIO-A® Tissue Reinforcement is intended for use in the reinforcement of soft tissue. GORE® BIO-A® Tissue Reinforcement may be used include hernia repair as suture-line reinforcement, muscle flap reinforcement, and general tissue reconstructions. Document
Document
Advances in Bioabsorbable Implants for Abdominal Wall Reconstruction: Utility of GORE® ENFORM Biomaterial
GORE® ENFORM Biomaterial is a reinforcement for soft tissue composed of synthetic bioabsorbable polyglycolic acid and trimethylene carbonate which has proven to be effective for hernia repair.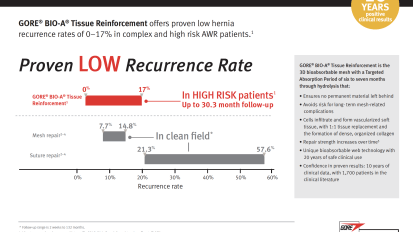 Document
Document
Better AWR Outcomes. Reinforced by Data. GORE® BIO-A® Tissue Reinforcement offers low complication rates in complex and high-risk patients
GORE® BIO-A® Tissue Reinforcement offers proven low hernia recurrence rates of 0–17% in complex and high risk AWR patients. Document
Document
General Surgery News Special Report: Clinical Discussion of Advances in Tissue Reinforcement for Abdominal Wall Reconstruction
In early 2021, as part of the Gore Medical Mastery Series, a faculty of surgeons convened in a virtual hybrid symposium in the midst of the COVID-19 pandemic to discuss their approaches to AWR, hernia repair, and tissue reinforcement. Video
Video
Patient quality of life with innovative biomaterials: Clinical review with over seven years of follow up
John Scott, MD, FACS, provides a post-operative clinical review of patients' quality of life after a hernia repair.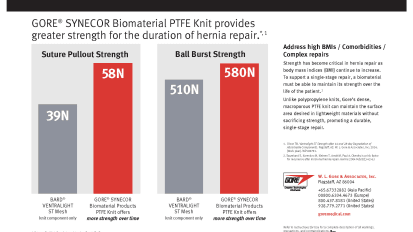 Document
Document
28-Day Strength Charts: GORE® SYNECOR Intraperitoneal Biomaterial
GORE® SYNECOR Biomaterial PTFE Knit provides greater strength for the duration of hernia repair. Document
Document
Better AWR Outcomes. Reinforced by Data. GORE® BIO-A® Tissue Reinforcement offers proven low complication rates in high risk AWR patients
GORE® BIO-A® Tissue Reinforcement offers proven low complication rates in high risk AWR patients1 vs. BARD® DAVOL PHASIX Mesh. Video
Video
Robotic Sugarbaker Repair of a Parastomal Hernia utilizing GORE® SYNECOR Intraperitoneal Biomaterial
Karl A. LeBlanc, MD, MBA, FACS, FASMBS performs a Sugarbaker Repair of a Parastomal Hernia Utilizing GORE® SYNECOR Intraperitoneal Biomaterial 15 cm x 20 cm Synthetic Hybrid Mesh.